
In nature, self-assembled systems are key elements of living entities and, contrary to their synthetic counterparts, are usually out-of-equilibrium and multicomponent structures capable of dynamic behaviours such as reconfigurability, adaptation or evolution. They usually have, however, limited intrinsic reconfigurability, and producing the desired structures with more than a few different components is still highly challenging. Synthetic self-assembled materials are usually equilibrium structures resulting from the spatial organization of a repeating single component into a stable supramolecular assembly, such as micelles or colloidal crystals, with a prescribed set of useful properties. Self-assembly is a process whereby naturally occurring or rationally designed entities embed the necessary information to spontaneously interact and self-organize into functional superstructures of interest 1. This method expands the repertoire of shapes and functions attainable by isothermal self-assembly and creates a basis for adaptive nanomachines and nanostructure discovery by evolution. Strikingly, upon the appearance of a new energy minimum, DNA origamis isothermally shift from one initially stable shape to a radically different one, by massive exchange of their constitutive staple strands. It allows a given system to self-select its most stable shape in a large pool of competitive DNA strands. In situ, time-resolved observation reveals that this self-assembly is thermodynamically controlled, proceeds through multiple folding pathways and leads to highly reconfigurable nanostructures.

We show that, with a magnesium-free buffer containing NaCl, complex cocktails of DNA strands and proteins can self-assemble isothermally, at room or physiological temperature, into user-defined nanostructures, such as DNA origamis, single-stranded tile assemblies and nanogrids. 9.Thermal annealing is usually needed to direct the assembly of multiple complementary DNA strands into desired entities.Calculate the lattice energy for CaF 2.Įnergy of formation for one mole of CaF 2 from its elements = -1228 kJ/molīack to Periodic Trends and Ionic Compounds The sublimation energy for Sr is 164 kJ/mol, E i1 = +549.5 kJ/mol, E i2 = +1064.2 kJ/mol, E ea for Cl (g) = -348.6 kJ/mol, energy for the formation of one mole of SrCl 2 from its elements = -803.7 kJ/mol, and the bond dissociation energy for Cl 2 (g) = +243 kJ/mol.Įxercise 5. Which has the highest lattice energy, Na 3P or Na 2S?Įxercise 4. Order the following ionic compounds from lowest to highest lattice energy. What is the lattice energy for MgF 2?Įxercise 2. Consider the following Born-Haber cycle for MgF 2. Worksheet: Ionic Compounds, Born-Haber Cycle, and Lattice EnergyĮxercise 1.

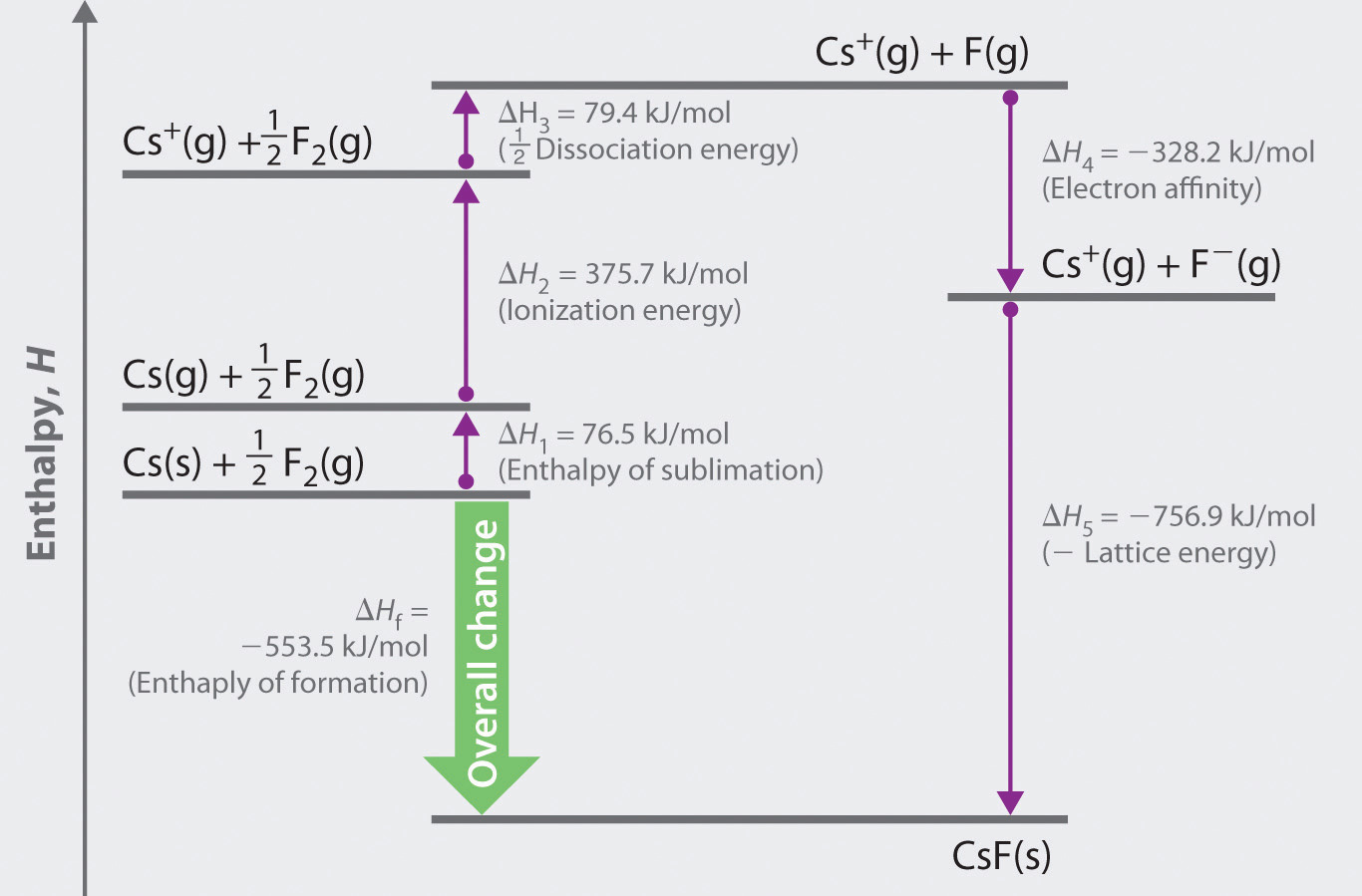
A more extensive list can be found in other tables or in the Handbook of Physics and Chemistry. In the case of NaCl and MgCl 2, the MgCl 2 has the larger lattice energy because the magnesium cation is smaller, and the charge is a +2 rather than a +1 for the sodium ion.īelow is a table with the lattice energies of some ionic compounds. This is because the charges are the same, but the potassium ion is larger than the sodium ion. For example, the lattice energy of NaCl is larger than the lattice energy of KCl. Lattice energies are large when ions are closer together, the distance between the ions is small and when the charges are larger. Coulomb’s law is equal to a constant, k, multiplied by the product of the ion charges, z 1 and z 22, between the ions. Recall, lattice energy is positive meaning it is endothermic. The stronger the bond, the higher the lattice energy. Lattice energy, E lattice is dependent on the strength of the bond between the cation and anion in an ionic bond. The lattice energy is always positive, because it takes energy to separate the ions from the solid. The equation for the lattice energy is the reverse of the equation in Step 5 in the figure below, for the formation of the solid from its ions which releases 787 kJ/mol of energy.Ī Born-Haber cycle allows the calculation of the lattice energy for a solid ionic compound. The process absorbs energy, and is highly endothermic. Lattice energy, E lattice is the energy required to separate one mole of a solid ionic compound into its gaseous ions.


 0 kommentar(er)
0 kommentar(er)
